Let’s Get to It:
What the Intracept™ Procedure Involves
The Intracept Procedure is a minimally invasive, outpatient procedure for patients with vertebrogenic pain. The procedure targets a specific nerve within the vertebra called the basivertebral nerve and has been shown to improve function and relieve pain long-term. The procedure is implant-free, preserving future treatment options for other spine conditions.
Here’s what the procedure involves.
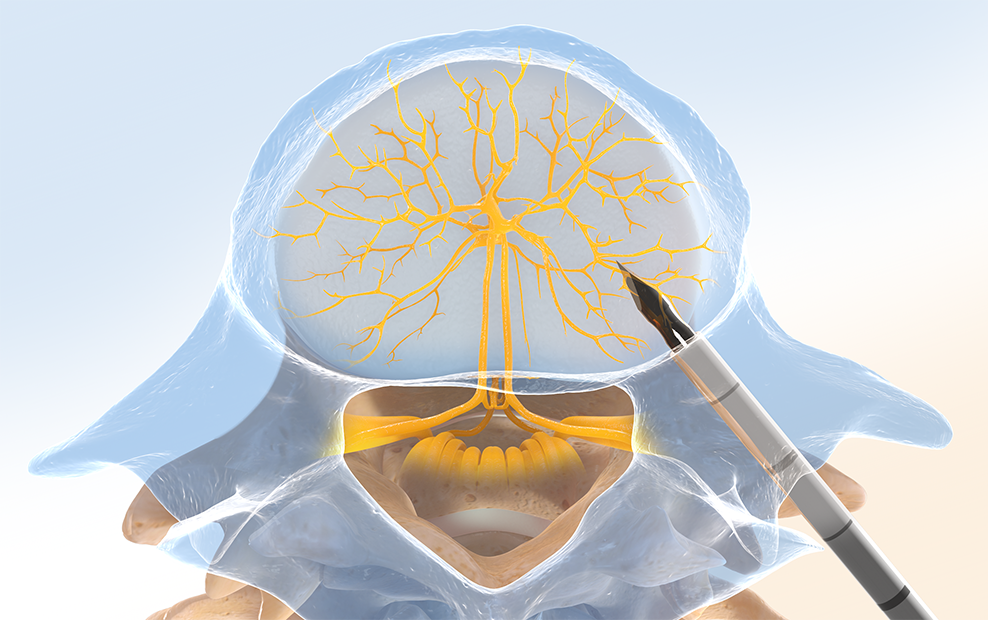
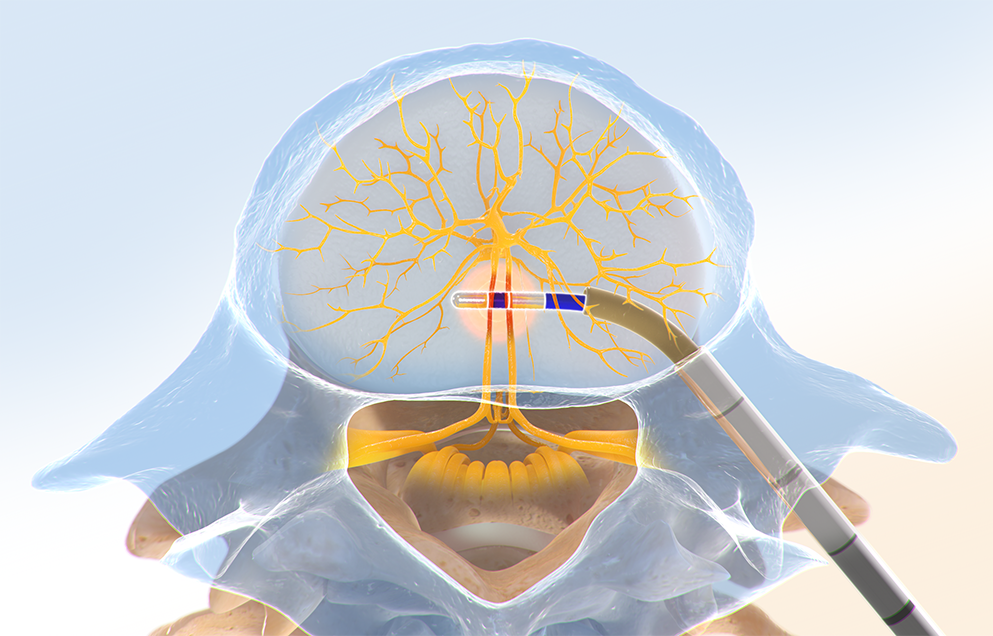
Step One: Access the Pedicle
Under fluoroscopic guidance, the Intracept™ Introducer Cannula Assembly is advanced through the pedicle.
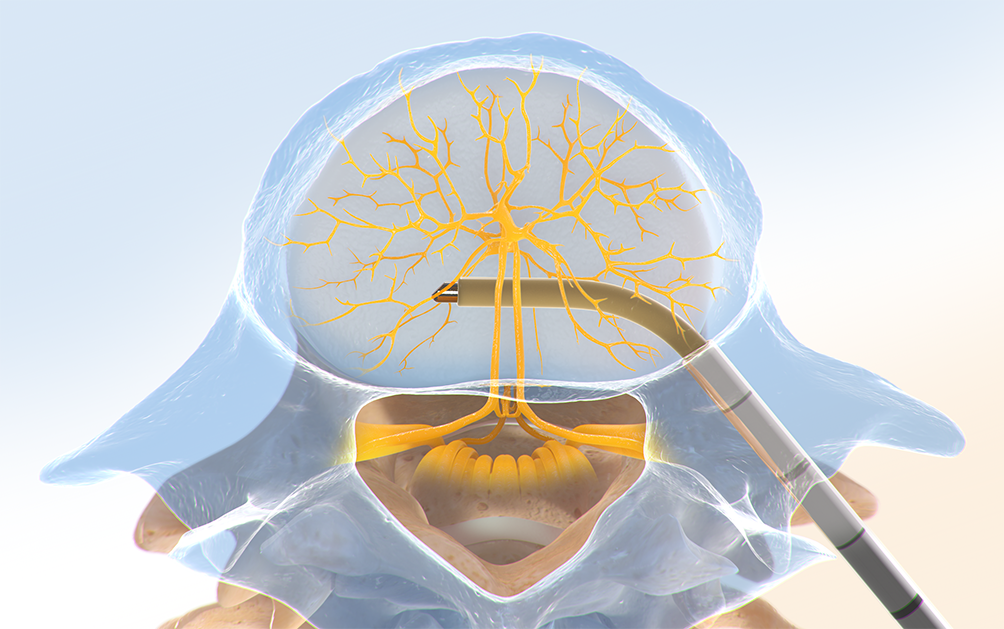
Step Two: Create the Channel
The Intracept™ Curved Cannula Assembly is used to create a channel to the trunk of the basivertebral nerve.
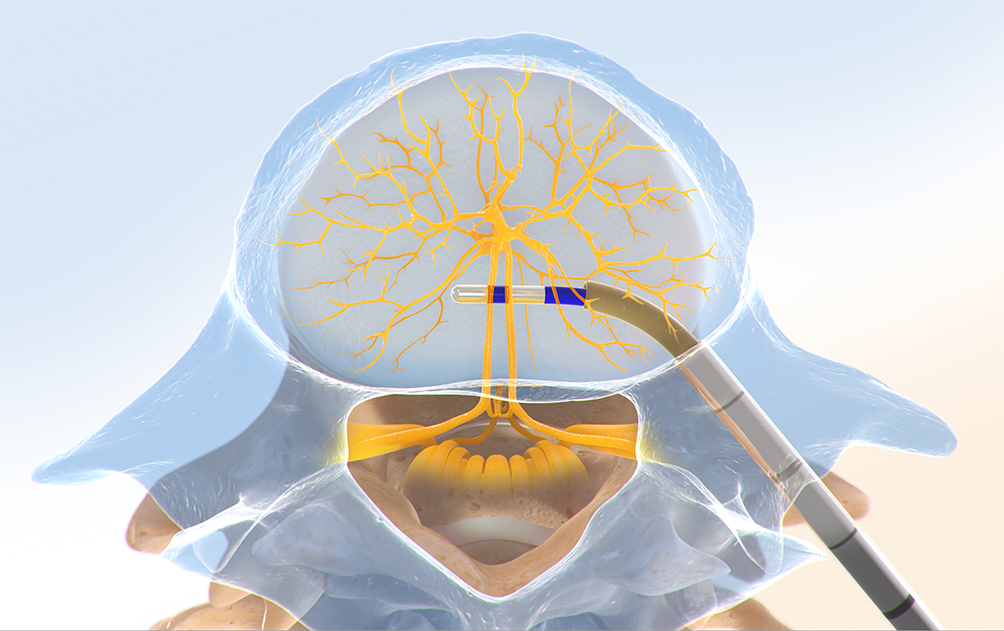
Step Three: Place the RF Probe
The Intracept™ RF Probe is inserted into the curved path and placed at the trunk of the basivertebral nerve.
Step Four: Ablate the BVN
The Intracept™ RF Generator is used to deliver radiofrequency energy that ablates the basivertebral nerve.
Watch a Procedure Walkthrough
Watch an Intracept™ Procedure with discussion featuring Dr. Greg Moore.
The Intracept™ System:
Now with Next-Generation Access Instruments
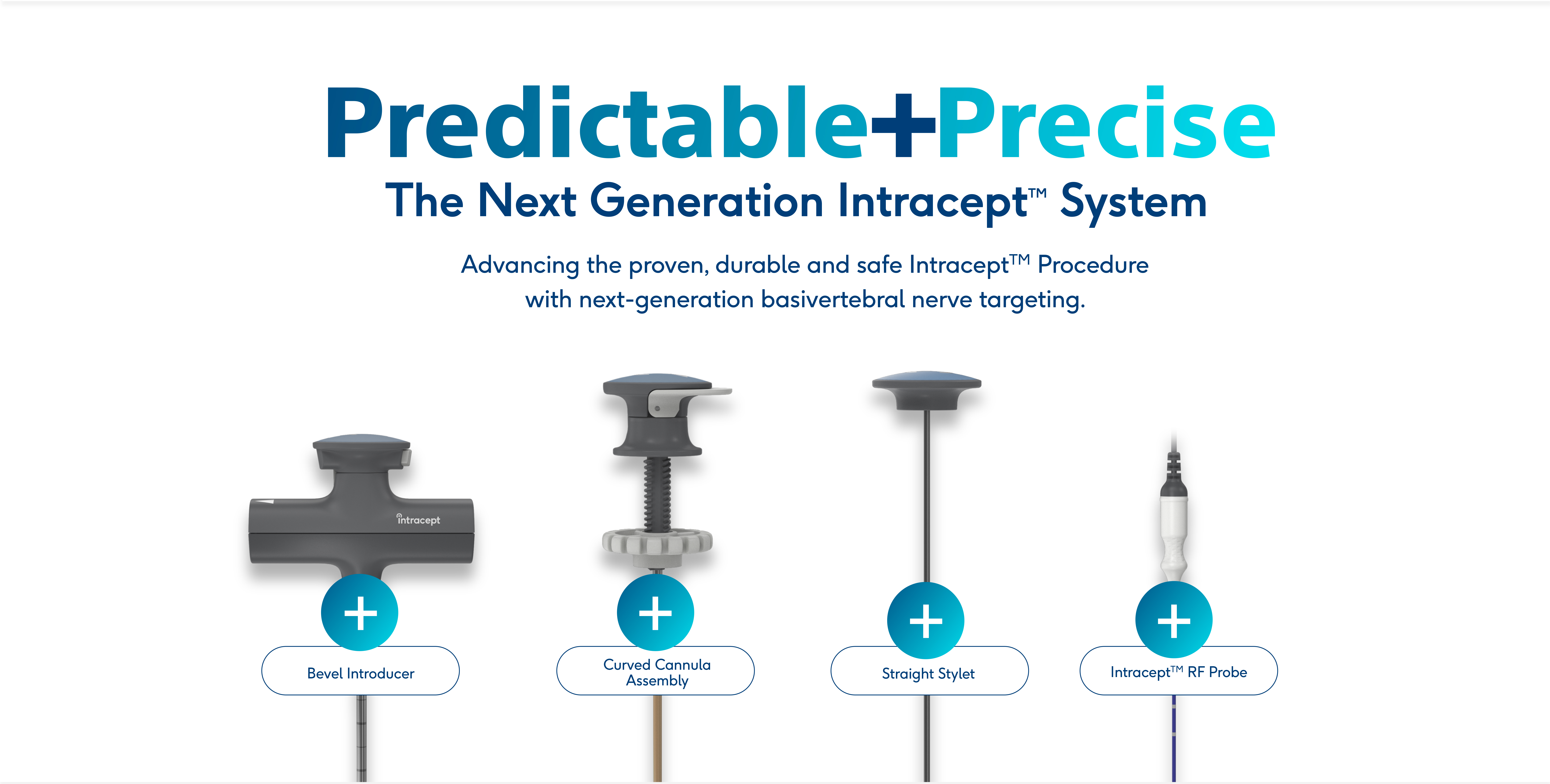
The proven, durable and safe Intracept™ Procedure now features next-generation Intracept Access Instruments for predictable and precise targeting of the basivertebral nerve.
What Physicians Are Saying About Intracept
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. See important safety information below.
Physicians: See Indications, Contraindications, and Risks
Indications for Use: The Intracept™ Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change). Contraindications - Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in: Patients with severe cardiac or pulmonary compromise, patients with active implantable pulse generators (e.g. pacemakers, defibrillators), patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal), patients with active systemic infection or local infection in the area to be treated, patients who are pregnant, and/or skeletally immature patients (generally ≤ 18 years of age). Refer to the Instructions for Use provided with the Intracept Procedure or www.relievant.com/intracept/ for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
Patients: See the Indications & Risks Involved
Contraindications include being pregnant, having weakened cardiac or pulmonary function, having an active implanted electronic medical device in the body (such as a pacemaker or defibrillator), being diagnosed with a systemic or local infection, or having an anatomy that could be damaged unintentionally while ablating the basivertebral nerve (based on your physicians’ clinical review). The Intracept Procedure is also contraindicated in patients who are skeletally immature – which generally means individuals under the age of 18 are not candidates. There are also certain risks and precautions regarding the procedure which you should be aware of before proceeding. Talk with your doctor about what indicates, and contraindicates, certain patients for the Intracept Procedure – as well as the risks and precautions for the procedure. For complete indications for use, contraindications, warnings, precautions, and side effects visit www.relievant.com/intracept/.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.