
Advancing the proven, durable and safe Intracept® Procedure with next-generation basivertebral nerve targeting.
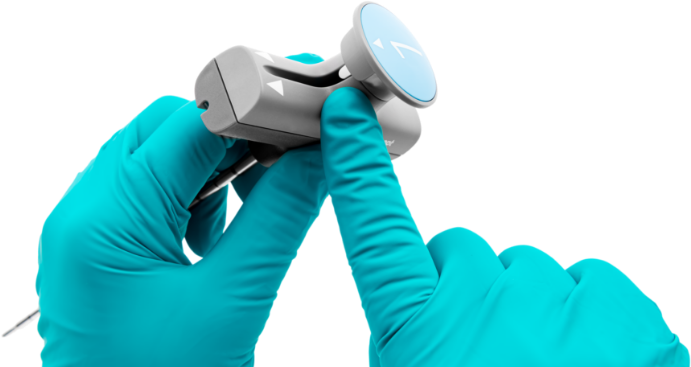
Bevel and Diamond Introducers
Predictable
Reliable performance through a wide range of bone densities.
Precise
Controlled pedicle access.
The Bevel and Diamond Introducers are used to access the pedicle.
- Ergonomic handle enables smooth instrument exchange
- Integrated leverage ramp on handle eases stylet removal
- Scallop feature on stylet tip provides depth marker for optimal positioning and relief for compacted bone material to further enable smooth instrument exchange


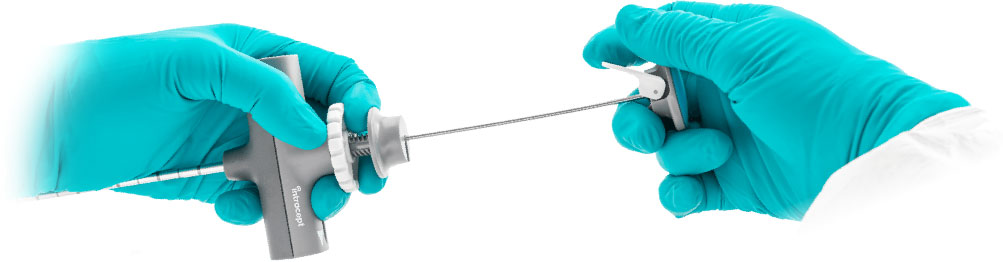
Curved Cannula Assembly
Predictable
Consistent performance even in hard bone.
Precise
True steerability allows for controlled targeting adjustments.
The Curved Cannula Assembly includes the J-Stylet and creates a predictable curved access path to enable precise targeting of the basivertebral nerve.
- Gear wheel with triple thread allows for quick positioning
- Bail feature enables smooth instrument exchange

- The J-Stylet’s D-shape cross-section enables predictable performance and steerability – even in hard bone

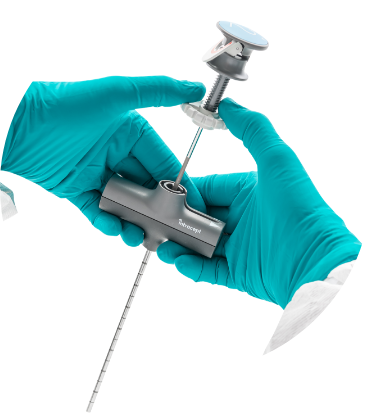
Straight Stylet
Predictable
Efficiently extends access path for consistent targeting.
Precise
Full radiopacity for positioning.
The Straight Stylet extends or changes access path trajectory to accurately target the basivertebral nerve.
- Bullet nose tip eases penetration through bone
- Fully radiopaque design enables easy visualization
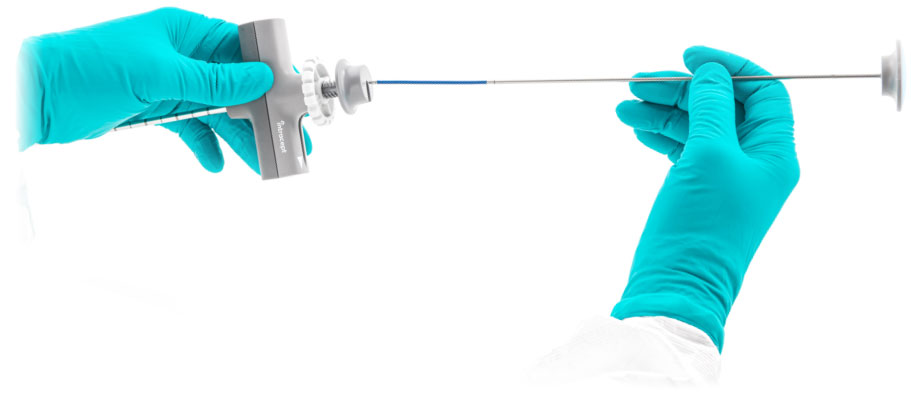
Intracept® RF Probe
Predictable
Temperature controlled bi-polar RF energy provides effective ablation zone formation.
Precise
Two radiopaque electrodes allow for accurate positioning.
The flexible Intracept RF Probe allows for optimal placement and targeting of the basivertebral nerve.
- Two sets of ablation parameters enable treatment options based on probe position
- Flexible shaft increases C-arm operating clearance

Intracept® RF Generator

The Intracept RF Generator is specifically designed to ablate the basivertebral nerve. The RF technology features:
- Intuitive step-by-step user interface to simplify set-up and delivery of ablation treatment
- Real-time power management to regulate temperature and ramp rate to ensure an efficient ablation
- Two sets of ablation parameters to enable treatment options based on Intracept® RF Probe position
Comprehensive Training and Support
Physicians performing the Intracept® Procedure are provided with comprehensive training and support. This includes pre- and post-training resources and procedural support to enable physicians to gain the knowledge and experience necessary to perform the Intracept® Procedure safely and effectively.
- In-person comprehensive training program designed and delivered by physicians experienced in performing the Intracept Procedure
- Case support from experienced Relievant clinical specialists committed to patient outcomes and safety
- Comprehensive program development support, including materials to aid in patient identification, build procedural expertise, share best practices, and facilitate peer-to-peer collaboration
See How It All Comes Together: The Intracept® System
The Intracept System enables a physician to effectively target and ablate the basivertebral nerve (BVN) to provide relief of vertebrogenic chronic lower back pain (CLBP). The Intracept System includes purpose-built Intracept® Access Instruments for creating a path to the BVN, proprietary radiofrequency (RF) ablation technology for effectively ablating the BVN, and comprehensive training and case support.



