When Your Pain Is Finally Defined.
That’s Living Proof.
Vertebrogenic Pain Is Understood
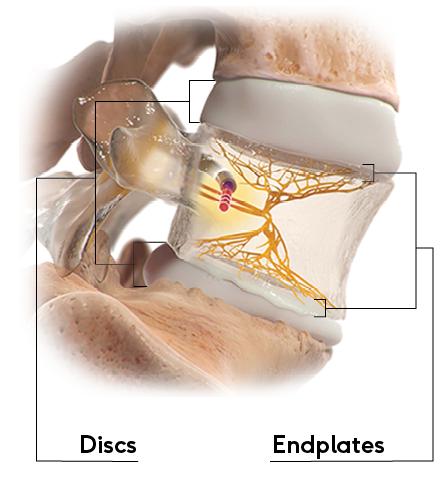
How Patients Describe Vertebrogenic Pain
The disc and endplate are both part of the anterior spinal column and produce similar low back pain symptoms. However, endplate pain is associated with distinctive changes on routine MRI called Modic changes.
Patients who find relief from the Intracept™ Procedure often describe pain in the middle of their low back that is made worse by physical activity, prolonged sitting, and bending forward, or with bending and lifting.1
Vertebrogenic Pain Has a Clear Diagnosis
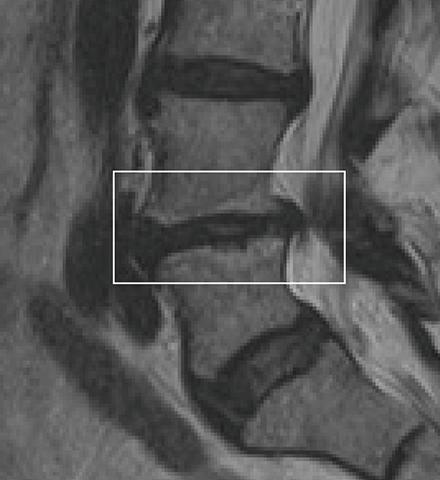
To confirm that a patient has vertebrogenic pain, physicians use MRI to look for specific changes that occur with endplate inflammation, which are called Modic changes.
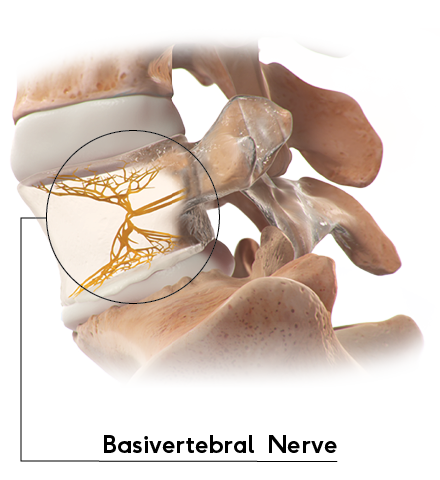
Ablating the Basivertebral Nerve Is the Key
The basivertebral nerve enters through an opening in the back of the vertebral body and branches near the center of the vertebral body, sending nerves to innervate the superior and inferior endplates. These nerve endings transmit pain signals from the endplate to the brain and have been shown to increase in number with endplate damage or degeneration. 2-4
The Latest Living Proof
Boston Scientific Introduces the Intracept™ Intraosseous Nerve Ablation System for Vertebrogenic Low Back Pain in Europe
The first commercial Intracept Procedure was performed by a team at Stoke Mandeville Hospital, Buckinghamshire Healthcare NHS Trust, in the United Kingdom led by Dr. David McKean, consultant musculoskeletal radiologist.
Now Enrolling – the IMPROVE Study for the Intracept™ Procedure
The Intracept Minimally-invasive PROcedure for VErtebrogenic back pain (IMPROVE) Study will strengthen evidence around global and real-world Intracept Procedure outcomes across multiple physician specialties and geographies.
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. See important safety information below.
Physicians: See Indications, Contraindications, and Risks
Indications for Use: The Intracept™ Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change). Contraindications - Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in: Patients with severe cardiac or pulmonary compromise, patients with active implantable pulse generators (e.g. pacemakers, defibrillators), patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal), patients with active systemic infection or local infection in the area to be treated, patients who are pregnant, and/or skeletally immature patients (generally ≤ 18 years of age). Refer to the Instructions for Use provided with the Intracept Procedure or www.relievant.com/intracept/ for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
Patients: See the Indications & Risks Involved
Contraindications include being pregnant, having weakened cardiac or pulmonary function, having an active implanted electronic medical device in the body (such as a pacemaker or defibrillator), being diagnosed with a systemic or local infection, or having an anatomy that could be damaged unintentionally while ablating the basivertebral nerve (based on your physicians’ clinical review). The Intracept Procedure is also contraindicated in patients who are skeletally immature – which generally means individuals under the age of 18 are not candidates. There are also certain risks and precautions regarding the procedure which you should be aware of before proceeding. Talk with your doctor about what indicates, and contraindicates, certain patients for the Intracept Procedure – as well as the risks and precautions for the procedure. For complete indications for use, contraindications, warnings, precautions, and side effects visit www.relievant.com/intracept/.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
- Koreckij T, Kreiner S, Khalil JG, Smuck M, Markman J, Garfin S. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. NASSJ. Published online October 26, 2021. DOI: https://doi.org/10.1016/j.xnsj.2021.100089
- Fras C, Kravetz P, Mody DR, Heggeness MH. Substance P-containing nerves within the human vertebral body: an immunohistochemical study of the basivertebral nerve. The Spine Journal: Official Journal of the North American Spine Society. 2003;3(1):63-7.
- Bailey JF, Liebenberg E, Degmetich S, Lotz JC. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. Journal of Anatomy 2011;218(3):263-70.
- Lotz JC, Fields AJ, Liebenberg EC. The Role of the Vertebral End Plate in Low Back Pain. Global Spine J 2013;03:153-64.
- Khalil, J., et al. Intraosseous basivertebral nerve ablation: 5-year outcomes from three long-term follow-up studies. Interventional Pain Medicine, Volume 3, Issue 4, 2024,100529, ISSN 2772-5944, https://doi.org/10.1016/j.inpm.2024.100529.doi.org/10.1007/s00586-020-06448-x
- Data on file.
NM-2011417-AA