Hero Curved Left No Overlay
Boston Scientific Introduces the Intracept™ Intraosseous Nerve Ablation System for Vertebrogenic Low Back Pain in Europe
Boston Scientific Corporation announced CE mark and introduction in Europe of the Intracept™ Intraosseous Nerve Ablation System. The Intracept system is the only treatment specifically designed to target the basivertebral nerve for the relief of chronic vertebrogenic...
Now Enrolling – the IMPROVE Study for the Intracept™ Procedure
Boston Scientific recently began enrollment in a new study – the Intracept Minimally-invasive PROcedure for VErtebrogenic back pain (IMPROVE) – to compile real-world outcomes of patients treated with the next-generation Intracept™ Access Instruments. The IMPROVE Study...
Boston Scientific Announces Positive Noridian Local Coverage Determination for the Intracept™ Procedure
Policy expands physician and patient access for vertebrogenic low back pain treatmentMARLBOROUGH, Mass., Feb. 22, 2024 – Boston Scientific Corporation (NYSE: BSX) has announced that Noridian Healthcare Solutions, a Medicare Administrative Contractor (MAC), published a...
Relievant Medsystems Announces Positive Coverage Policy from Humana for the Intracept Procedure
Third national coverage policy published in 2023 for proven vertebrogenic pain treatment MINNEAPOLIS – November 13, 2023 – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low back pain, today announced that Humana...
Contraindications, Warnings, and Precautions
PRECAUTIONS The Intracept Access Instruments and the Intracept RF Probe are single patient use only. The Introducer Cannula, Diamond Stylet, Bevel Stylet, J-Stylet, Straight Stylet, Drill, and Probe may be used to treat up to a maximum of four (4) vertebrae (L3, L4,...
Relievant Medsystems Announces Positive Coverage Policy for the Intracept Procedure from Anthem Blue Cross and Blue Shield
Expands access to vertebrogenic pain treatment for 36 million patients MINNEAPOLIS – October 3, 2023 – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low back pain, today announced that Anthem Blue Cross and Blue...
Relievant Medsystems Introduces Intracept Simulator System Powered by Medability for Improved and Expanded Physician Training Opportunities
Advanced technology enhances Intracept Procedure training for the treatment of vertebrogenic pain CHICAGO – IPSIS Annual Meeting – September 21, 2023 – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low back...
Relievant Medsystems Announces Pooled Analysis Demonstrating the Intracept Procedure Significantly Reduces Low Back Pain-related Healthcare Utilization
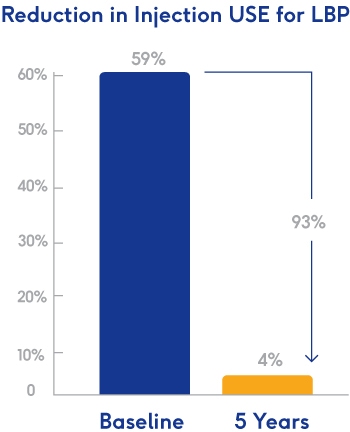
Findings show marked reductions in need for opioids and therapeutic lumbosacral spine injections five years post-procedure MINNEAPOLIS – September 12, 2023 – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low...
Relievant Medsystems Announces Pooled 4-Year Outcomes Validating Long-Term Effectiveness of the Intracept Procedure
Findings, Presented at Annual American Society of Pain & Neuroscience Conference, Demonstrate Significant and Durable Relief of Chronic Vertebrogenic Low Back Pain Minneapolis – July 20, 2023 – Relievant Medsystems, a company dedicated to transforming the...
Relievant Medsystems Introduces Next-Generation Access Instruments to Advance Intracept Procedure for Chronic Vertebrogenic Low Back Pain
Miami – ASPN Annual Conference – July 14, 2023 – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low back pain, today announced the release of next-generation Intracept® Access Instruments. The new instruments...
Hero Curved Left Overlay
Hero Curved Center
That’s Living
Proof.
Hero Curved Right
Welcome to the proof
of Intracept
Hero Image Text
Mattis Parturient Vestibulum Risus Sit
Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
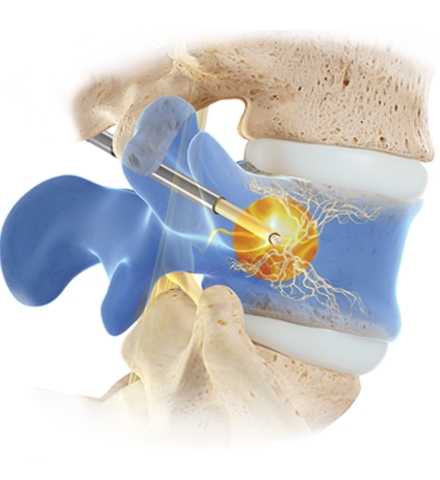
Image Text

Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Curabitur blandit tempus porttitor. Fusce dapibus, tellus ac cursus commodo, tortor mauris.
Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
Chart Text
Mattis Parturient Vestibulum Risus Sit
Key Findings:
Vulputate Pellentesque Lorem Adipiscing
Key Findings:
Vulputate Pellentesque Lorem Adipiscing
Video Text
Mattis Parturient Vestibulum Risus Sit
Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Curabitur blandit tempus porttitor. Fusce dapibus, tellus ac cursus commodo, tortor mauris.
3 Column Image
The Data Is Living Proof Too

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.

Fewer Opiods
Maecenas sed diam eget risus varius blandit sit amet non magna. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus.
Curve Right Text
Mattis Parturient Vestibulum Risus Sit
Curve Left Text
Mattis Parturient Vestibulum Risus Sit
Curve Left Text Overlay
Icon Lists
Accordion Cards
How we’re living the proof.
Now in Effect: New Category | CPT Codes
Intracept Long Term Outcomes
New Patient Story
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Indications (Global)
As with any surgical procedure, there are risks and considerations associated with the Intracept Procedure. See important safety information below.
Physicians: See Indications, Contraindications, and Risks
Indications for Use: The Intracept™ Intraosseous Nerve Ablation System is intended to be used in conjunction with radiofrequency (RF) generators for the ablation of basivertebral nerves of the L3 through S1 vertebrae for the relief of chronic low back pain of at least six months duration that has not responded to at least six months of conservative care, and is also accompanied by features consistent with Type 1 or Type 2 Modic changes on an MRI such as inflammation, edema, vertebral endplate changes, disruption and fissuring of the endplate, vascularized fibrous tissues within the adjacent marrow, hypointensive signals (Type 1 Modic change), and changes to the vertebral body marrow including replacement of normal bone marrow by fat, and hyperintensive signals (Type 2 Modic change). Contraindications - Use of the Intracept Intraosseous Nerve Ablation System is contraindicated in: Patients with severe cardiac or pulmonary compromise, patients with active implantable pulse generators (e.g. pacemakers, defibrillators), patients where the targeted ablation zone is < 10 mm away from a sensitive structure not intended to be ablated, including the vertebral foramen (spinal canal), patients with active systemic infection or local infection in the area to be treated, patients who are pregnant, and/or skeletally immature patients (generally ≤ 18 years of age). Refer to the Instructions for Use provided with the Intracept Procedure or www.relievant.com/intracept/ for potential adverse effects, warnings, and precautions prior to using this product.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
Patients: See the Indications & Risks Involved
Contraindications include being pregnant, having weakened cardiac or pulmonary function, having an active implanted electronic medical device in the body (such as a pacemaker or defibrillator), being diagnosed with a systemic or local infection, or having an anatomy that could be damaged unintentionally while ablating the basivertebral nerve (based on your physicians’ clinical review). The Intracept Procedure is also contraindicated in patients who are skeletally immature – which generally means individuals under the age of 18 are not candidates. There are also certain risks and precautions regarding the procedure which you should be aware of before proceeding. Talk with your doctor about what indicates, and contraindicates, certain patients for the Intracept Procedure – as well as the risks and precautions for the procedure. For complete indications for use, contraindications, warnings, precautions, and side effects visit www.relievant.com/intracept/.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Copyright © 2025 by Boston Scientific Corporation or its affiliates. All rights reserved.
Accordion List
Physicians
Curabitur blandit tempus porttitor. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum. Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
Intracept Long Term Outcomes
Curabitur blandit tempus porttitor. Aenean eu leo quam. Pellentesque ornare sem lacinia quam venenatis vestibulum. Morbi leo risus, porta ac consectetur ac, vestibulum at eros.
New Patient Story
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Carousel Doctor
What Physicians Are Saying About Intracept
Carousel Video
Looking for proof?
Hear from the ones living it.
Video Grid
Story
Debbie’s Story
Multiple injections, multiple failed
approaches—nothing worked.
Nullam quis risus eget urna mollis ornare vel eu leo. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Donec ullamcorper nulla non metus auctor fringilla. Cras justo odio, dapibus ac facilisis in, egestas eget quam.
Duis mollis, est non commodo luctus, nisi erat porttitor ligula, eget lacinia odio sem nec elit. Donec sed odio dui. Curabitur blandit tempus porttitor. Nullam quis risus eget urna mollis ornare vel eu leo. Etiam porta sem malesuada magna mollis euismod. Curabitur blandit tempus porttitor.
Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Cras justo odio, dapibus ac facilisis in, egestas eget quam. Vivamus sagittis lacus vel augue laoreet rutrum faucibus dolor auctor. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Sed posuere consectetur est at lobortis.
Next Step forms
Access Instruments CTA
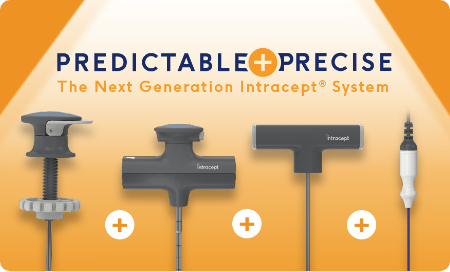
The Intracept® System:
Now with Next-Generation Access Instruments