Sustained Relief
At 24 Months1
Purpose
To report expanded outcomes and results for the INTRACEPT Study treatment arm.
Study Design
- The INTRACEPT Study randomized 140 patients (66 BVN ablation vs 74 SC) across 20 sites. Enrollment was stopped early due to statistical superiority at the pre-specified interim analysis; as a result, the control arm was offered early active treatment. Primary results were published in 2019, showing a statistically significant difference between the Intracept arm and the standard care arm for the primary endpoint and all secondary endpoints at three months post-procedure.
- The publication reports outcomes for 58 patients (88% retention rate) in the Intracept treatment arm that completed a 24-month follow up visit.
Key Findings
Patient Characteristics
Typical of anterior column pain, two-thirds of the patients presented with midline axial low back pain that was exacerbated with sitting, forward flexion and with position changes such as sitting to standing.
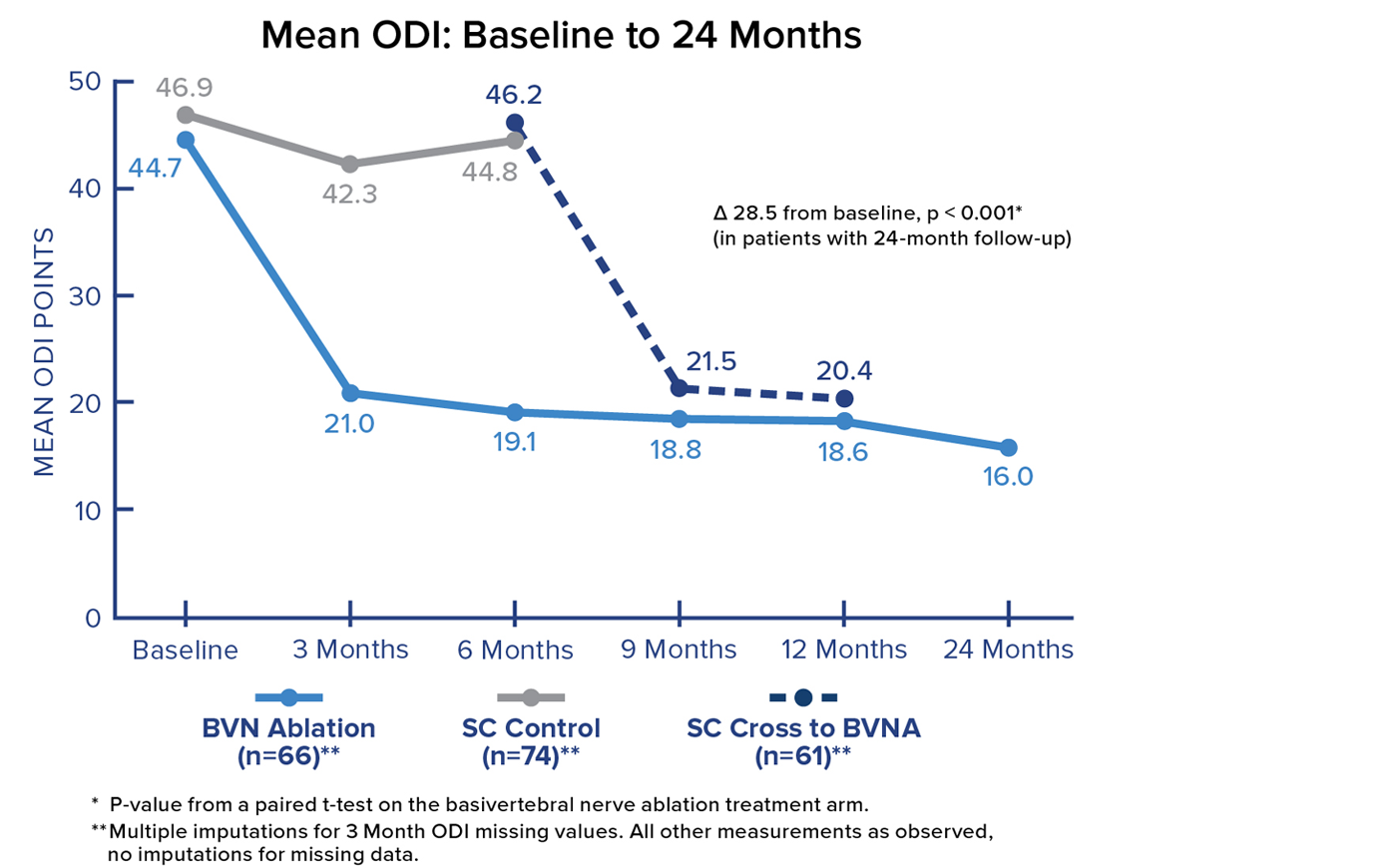
Mean ODI at 24 Months
Patients in the Intracept Procedure treatment arm realized a 28.5 point (p<0.001) reduction in mean Oswestry Disability Index (ODI) from baseline to 24 months. Significant and sustained reductions in mean ODI were demonstrated after treatment in all BVN ablation patients.
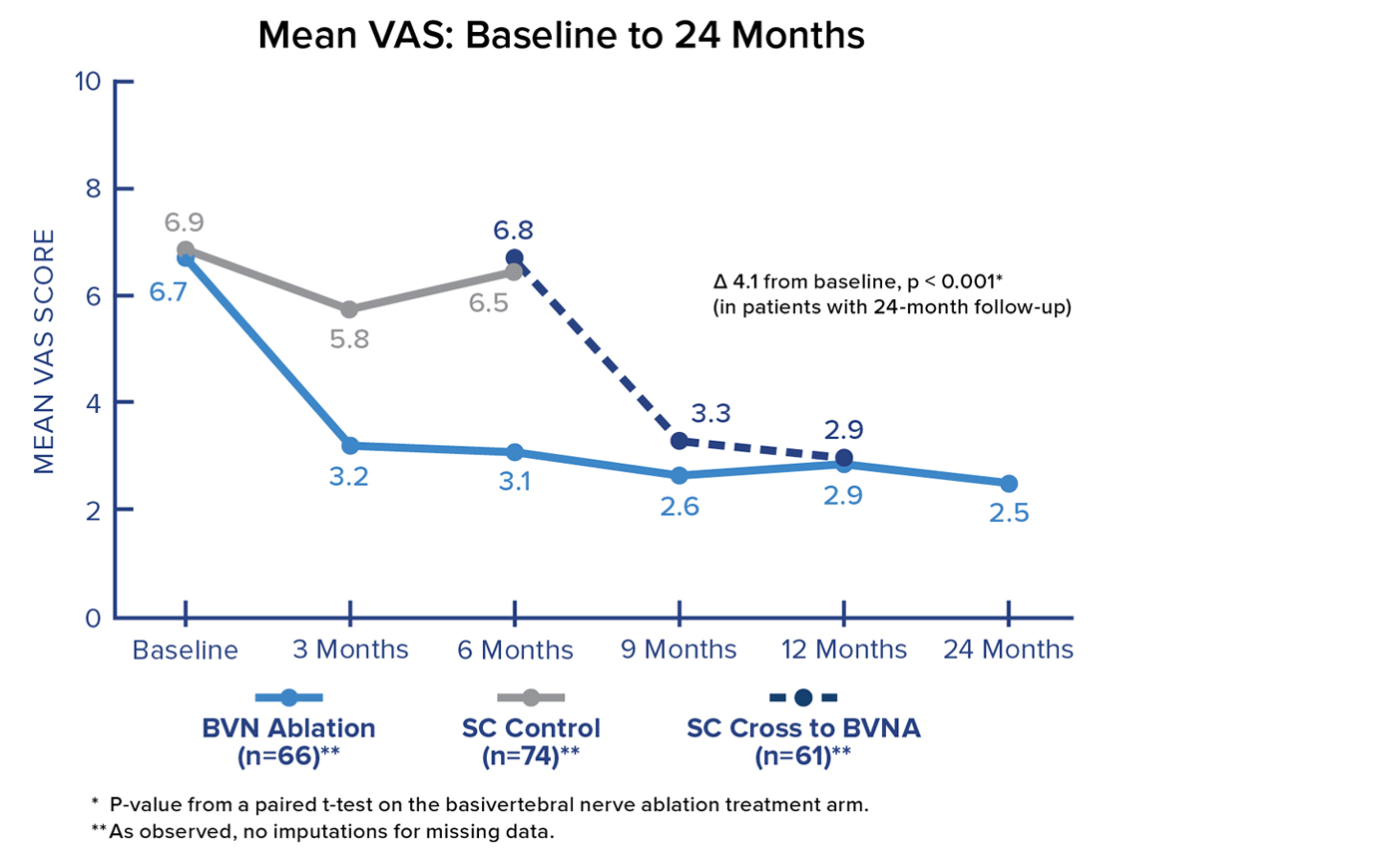
VAS Score
Patients in the Intracept treatment arm realized a 4.1-point reduction in mean Visual Analogue Scale (VAS) from baseline to 24 months. Significant and sustained reductions in mean VAS were demonstrated after treatment in all BVN ablation patients.
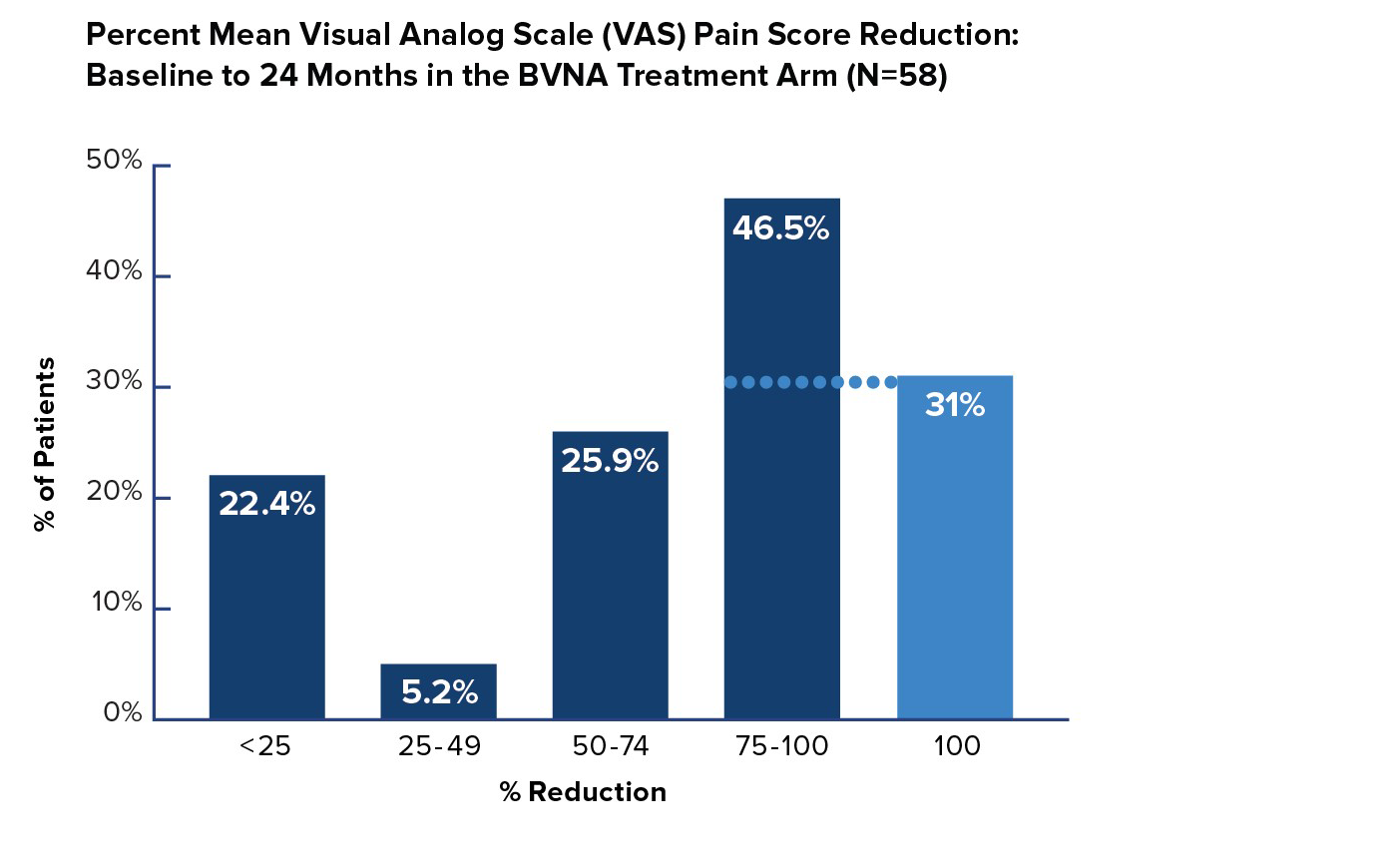
VAS Pain Reduction by Quadrant Improvement
In the Intracept treatment arm, 72% of patients reported a greater than 50% reduction in pain from baseline – and 31% of patients were pain-free 24 months after BVN ablation treatment.
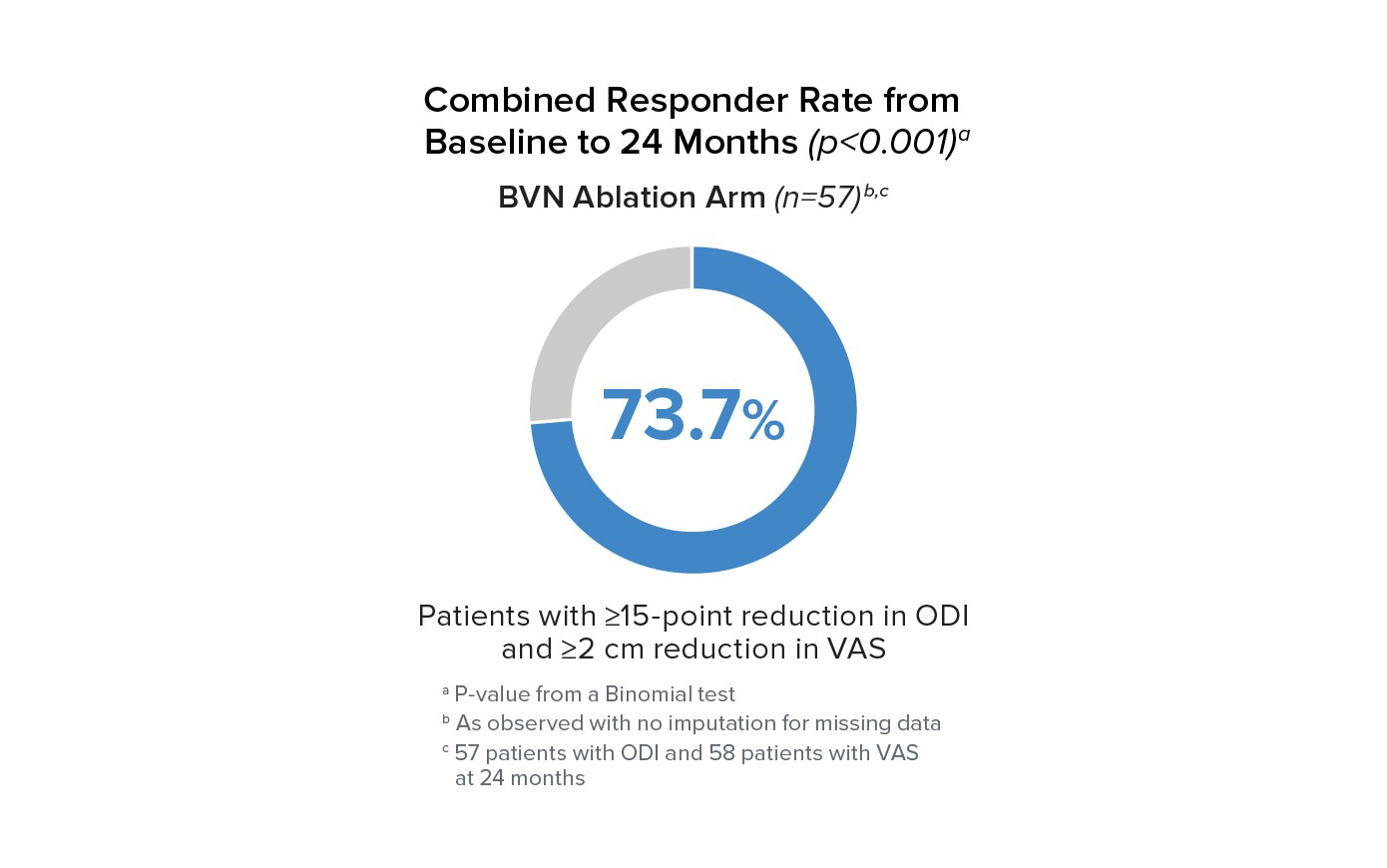
Responder Rates to 24 Months
Responder rates were significant compared to baseline at all timepoints for BVN ablation arm patients. At 24 months, 73.7% of patients reported a ≥15 point improvement in ODI and a ≥2 cm reduction in VAS.
Safety Results
There were no serious device or procedure-related events in BVN ablation-treated patients in the study.
Conclusion
Patients with chronic low back pain of vertebrogenic origin treated with BVN ablation exhibited significant improvements from baseline in measurements of pain, function, and quality of life at all follow-up timepoints through 24 months. Results remain consistent with prior studies and further validate Intracept safety, effectiveness, and long-term durability.
1. Koreckij T, Kreiner S, Khalil JG, Smuck M, Markman J, Garfin S. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-month treatment arm results. NASSJ. Published online October 26, 2021. DOI: https://doi.org/10.1016/j.xnsj.2021.100089